The “Big Picture”: For an overview of how our work on chromosomal stability connects to cellular disease states, check out our Research Overview page.
The big question: Now that we are thinking about cell stress pathways and how they respond to acute vs. chronic stressors, the question of how do cells “turn off” cell stress responses becomes important. Imagine that a cell stress response gets turned on but cannot be turned off. What would that do to the ability of cells to survive or respond to repeated exposure to stressors?
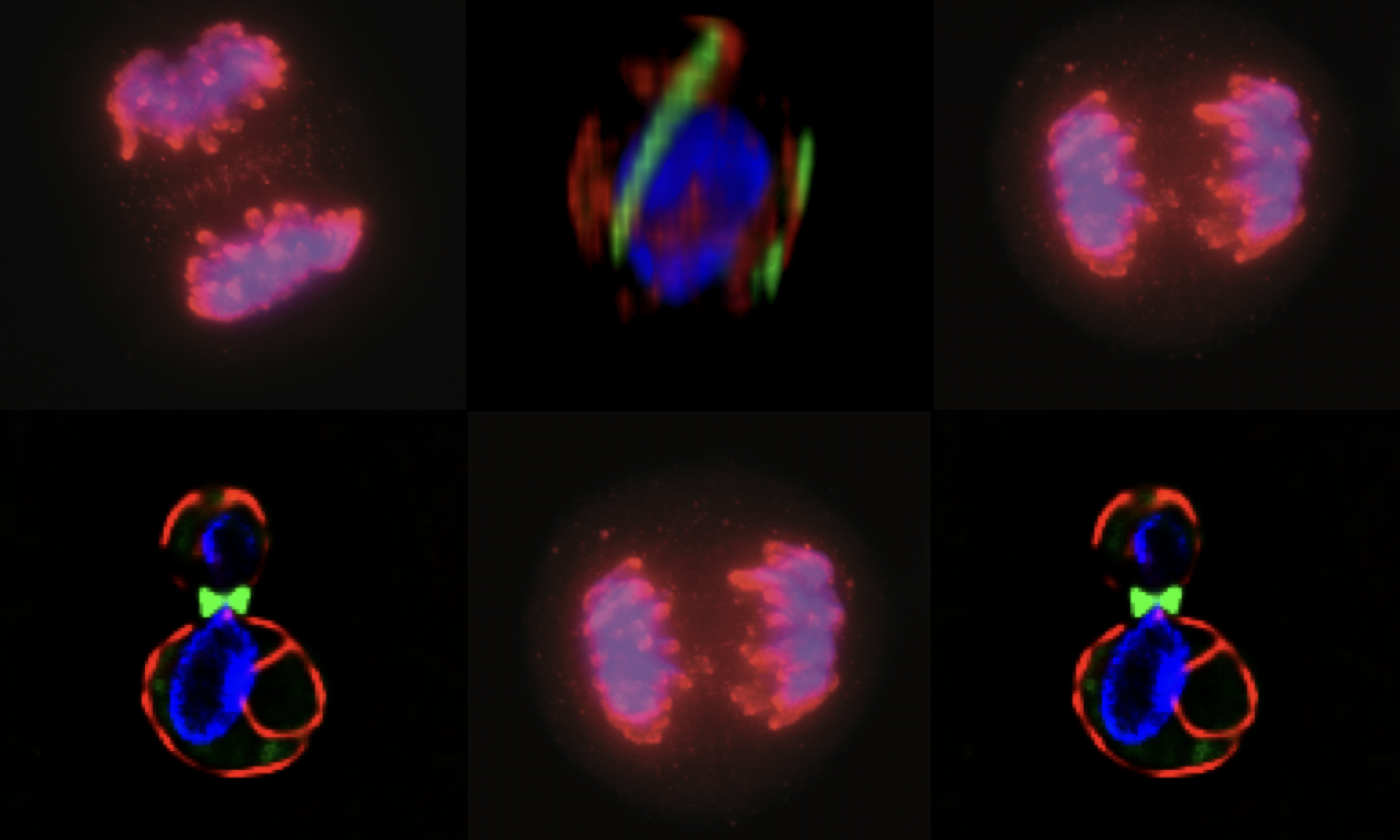
Regulation of macroautophagy at the cellular organelle level: We happened into this question after a serendipitous observation in the cell biology lab course, MCB140L (read the entire story here). Septins – a network of filaments that associate with cellular membranes – have been studied in the context of cytokinesis where they are believed to act to constrain abscission machinery at the site of cell division. Our observant student noted that cells that had been inadvertently starved rearranged their septins. Thus began a multiyear effort to characterize this rearrangement and investigate the hypothesis that this rearrangement has something to do with regulating autophagy.
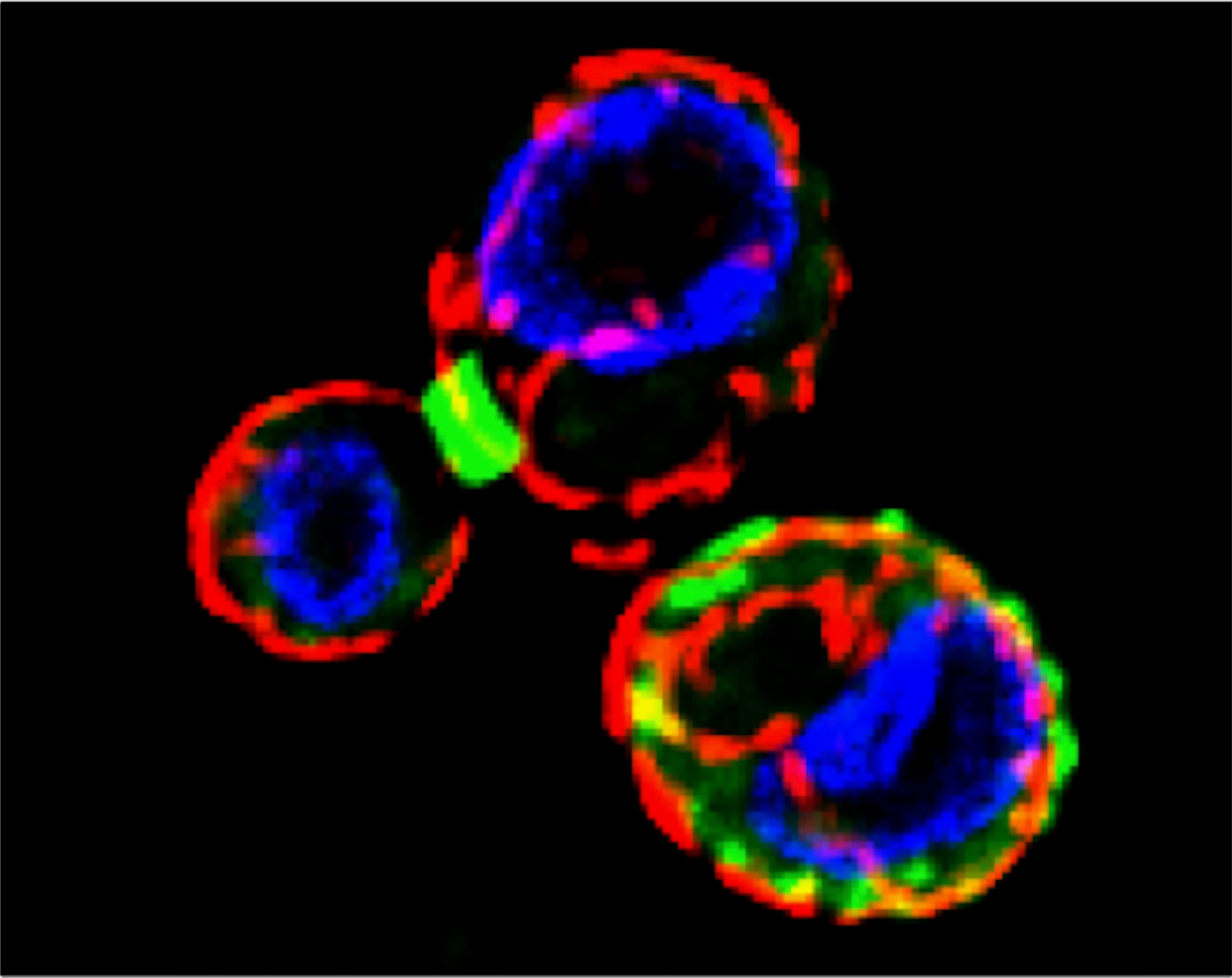
In work published recently, we demonstrated that the rearrangement of septins into cortical patches (see above) slowed the diffusion of autophagic membranes involved in delivering lipids to the autophagosome, the capture organelle that designates cargo for trafficking to the vacuole/lysosome. Mutations in septins fail to downregulate macroautophagy following nutrient deprivation (see below).
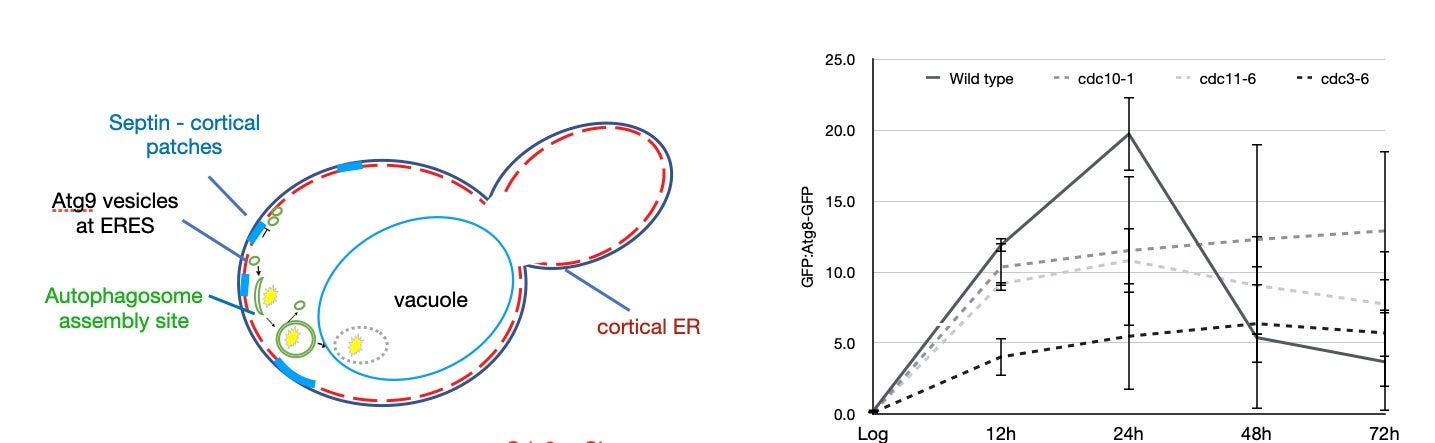
Next Steps: We are excited to use septin mutants that cannot downregulate macroautophagy as a model for investigating how dysregulated stress responses can impact cellular programs associated with disease states.