The “Big Picture”: For an overview of how our work on chromosomal stability connects to cellular disease states, check out our Research Overview page.
The big question: How are the final steps in cell division coordinated with the completion of DNA replication and the “resolution” of sister chromatids?
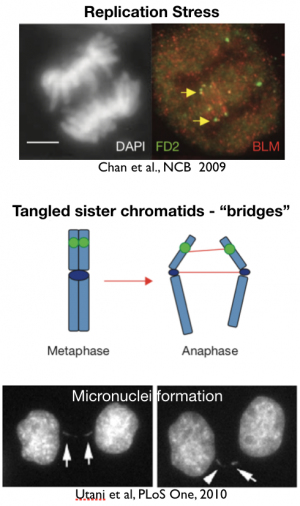
Early mitotic events (i.e., prior to anaphase) ensure that sister chromatids are properly aligned so that each daughter cell receives the same number of chromatids. Anaphase events must ensure that the final regions of the chromosome have replicated completely and that sister chromatids are “untangled” from one another to avoid chromosome damage. Work from Ian Hickson’s lab (and others; see figure below) highlight the importance of anaphase for maintaining chromosome integrity. In studies of patients’ cells with defects in a DNA helicase, BLM (Bloom’s syndrome helicase), Hickson and colleagues demonstrated that these cells are prone to chromosome damage and Bloom’s Syndrome patients exhibit a high incidence of cancer. The BLM helicase is critical for “untangling” sister chromatids in anaphase. Tangled sister chromatids are a natural consequence of the topological linkages formed in DNA during the replication process. These linkages can be made worse when cells experience “replication stress”. Unresolved sister chromatids (aka, lagging chromosomes) form anaphase “bridges” that if left unresolved can form micronuclei. Micronuclei are associated with high rates of genome rearrangement and with the most malignant forms of cancer.
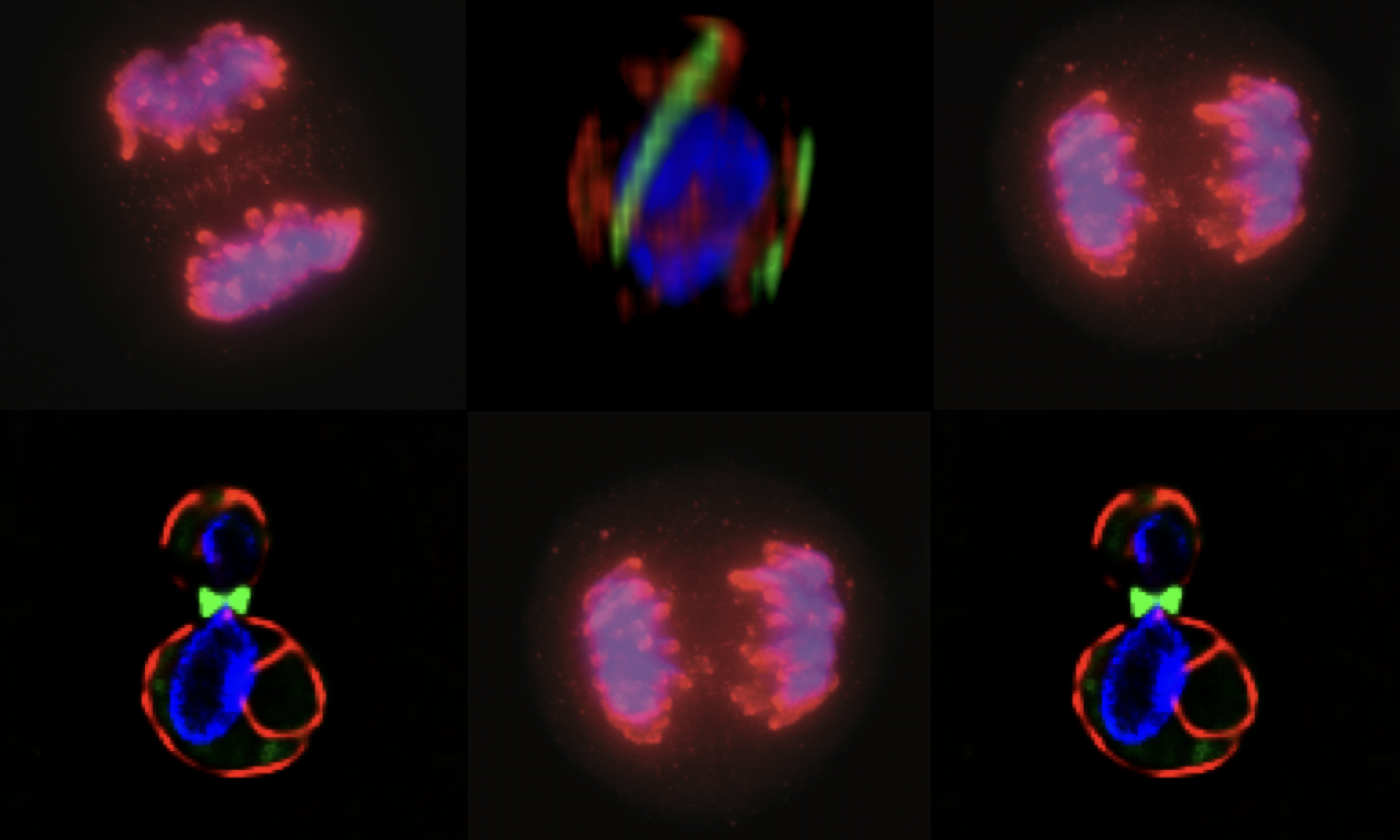
Anaphase surveillance: We propose that there are a series of regulatory pathways that link chromosome replication to anaphase events that ensure sister chromatid resolution and the maintenance of nuclear homeostasis. Our work (manuscript in preparation) argues that a nucleophagy pathway is activated under DNA replication stress and persists into anaphase. Evidence suggests that this pathway is involved in maintaining nuclear subdomains involved in such activities as DNA repair and ribosome biogenesis. More to follow!
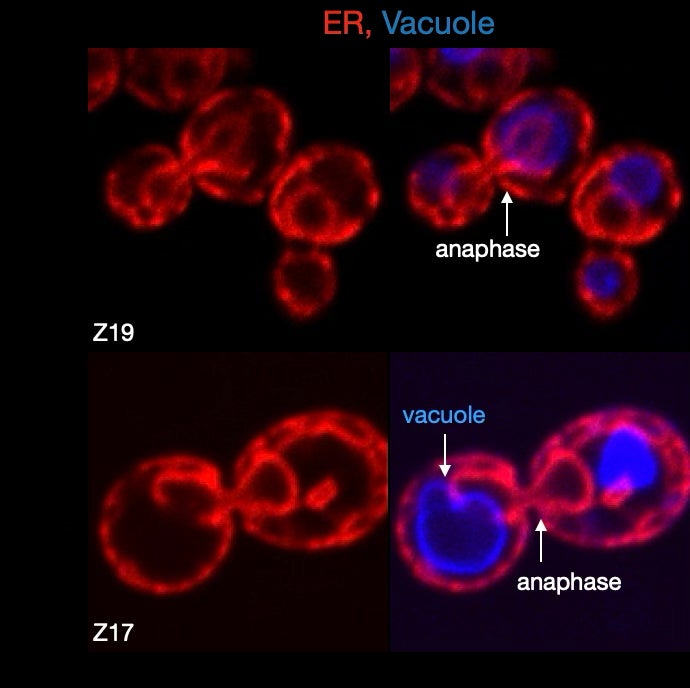
Replication Stress Induced Nucleophagy: Nucleophagy – autophagic pathways that use both microautophagy and macroautophagy mechanisms to target nuclear cargo to the vacuole/lysosome- have been described under nutrient deprivation conditions, nuclear pore stress and some evidence links these pathways to micronuclei formation after exposure to genome toxins. Our current work is focused on characterizing the nucleophagy pathways that respond to DNA replication stress, their relationship to cargo targeting, their importance in maintaining nuclear homeostasis and how chronic genomic stress alters their ability to maintain homeostasis.